
Learn how SuperLineage is used by major pharma to leverage RWD as core evidence in clinical trials
Scalable RWD validation via element-level lineage
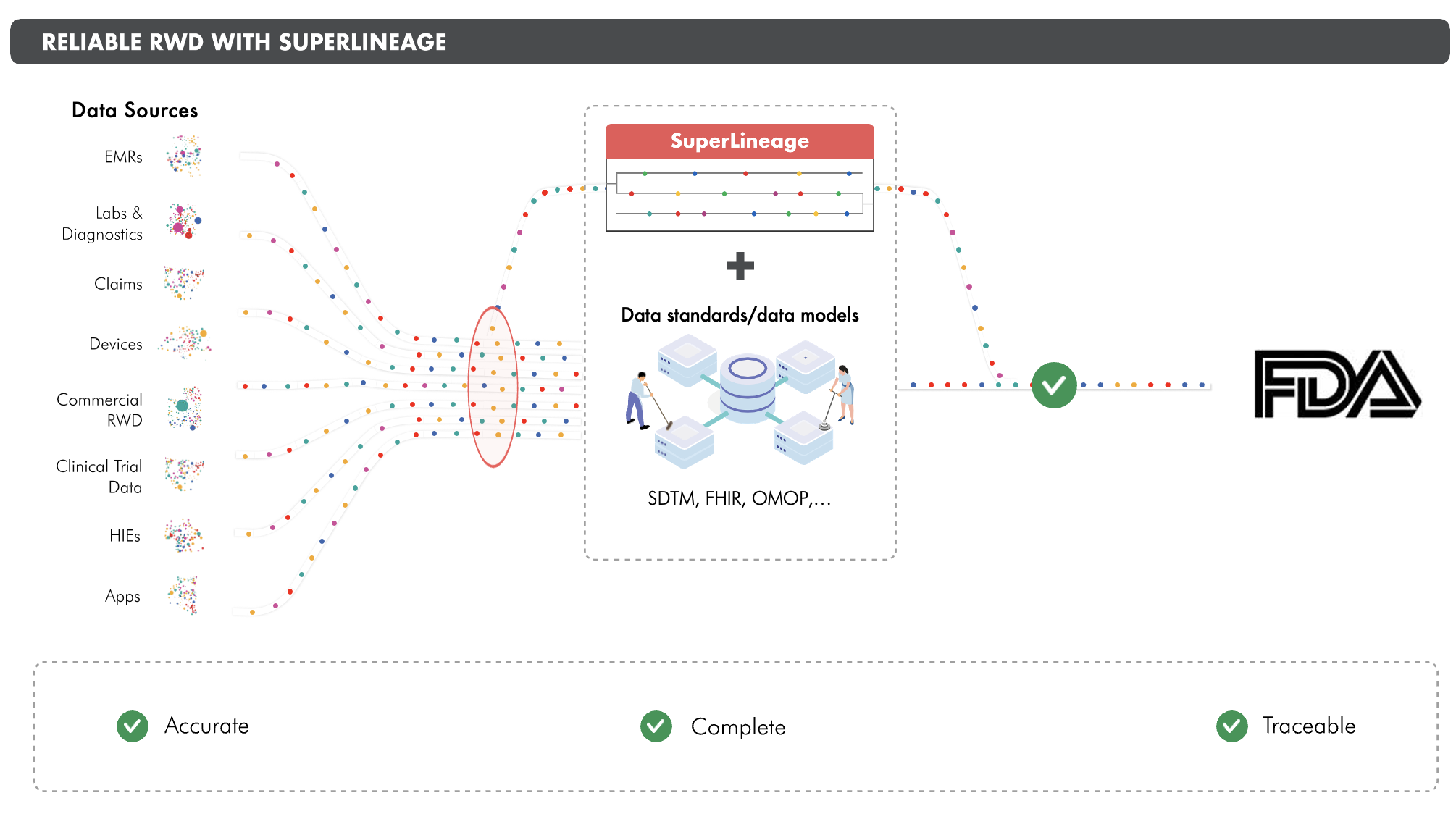
Droice Labs' SuperLineage is element-level lineage for RWD that enables validation of RWD for use in clinical trials.
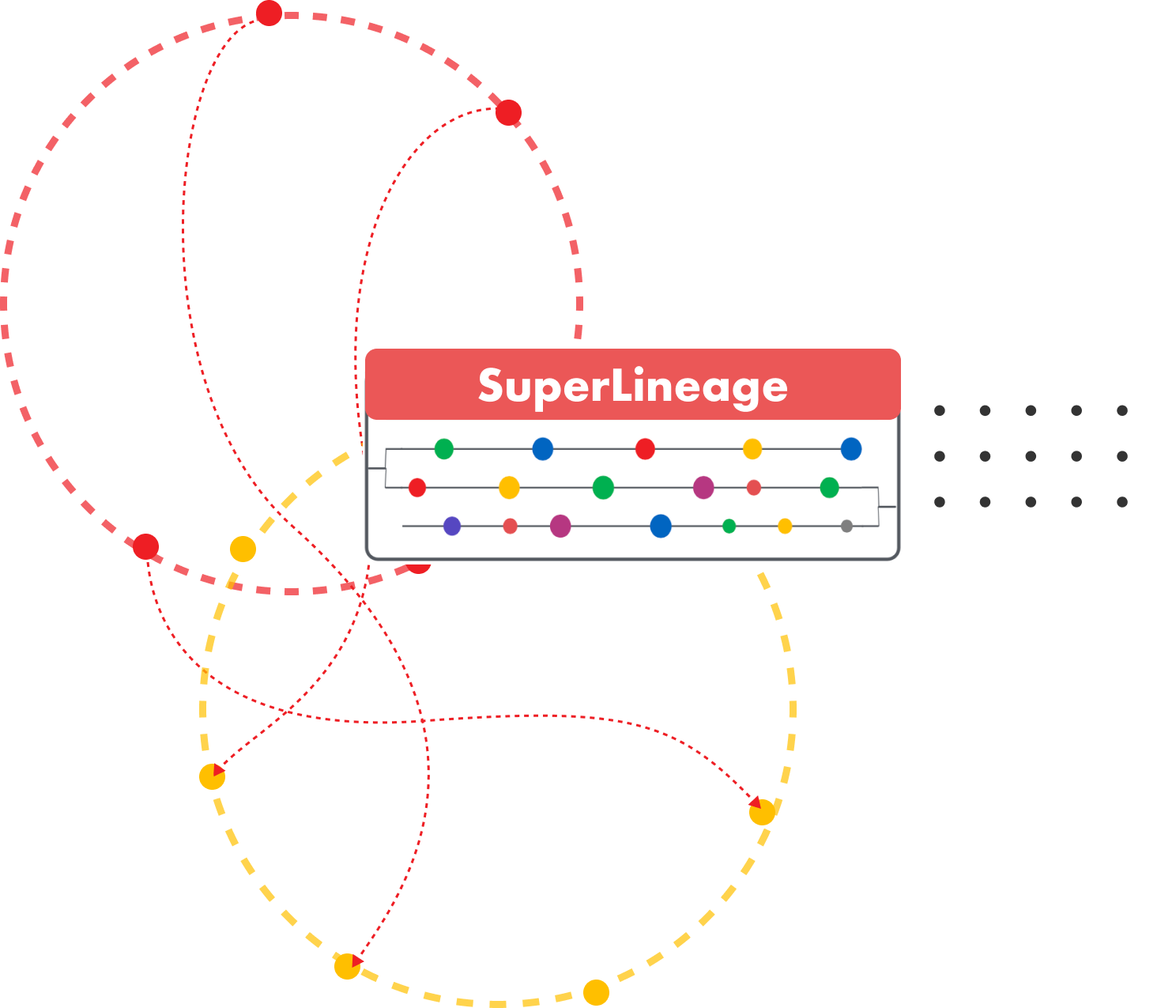
Why SuperLineage?
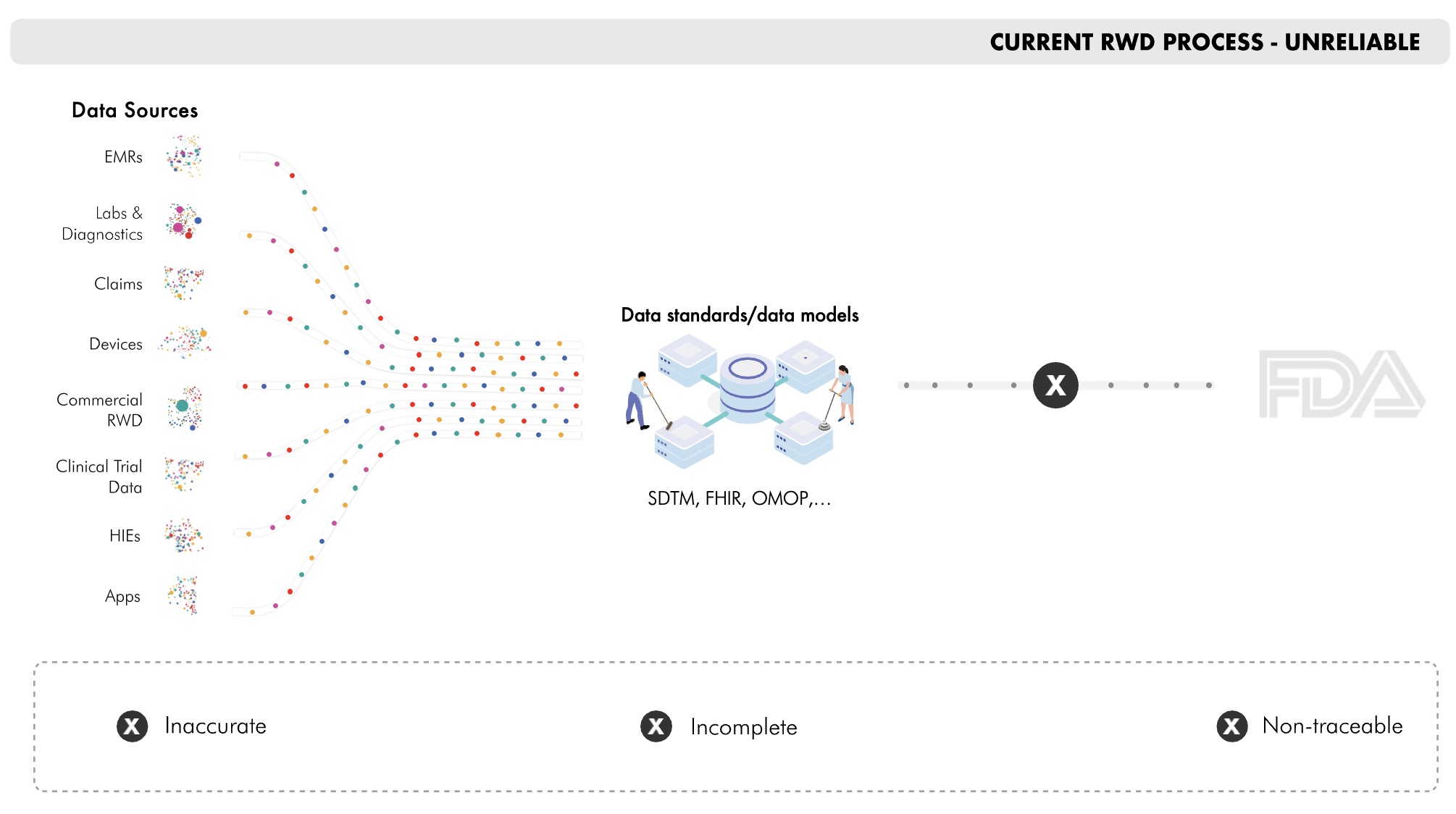
RWD suffers from data loss, which blocks its use in clinical trials. SuperLineage is the first traceability solution that scalably validates RWD by quantifying accuracy and completeness.
SuperLineage is standardized and can be supplied along with common data models & data standards like SDTM, OMOP, and FHIR.
Losslessly captures lineage for all stages of data transformation, from source to target and everything in between.

SuperLineage can be algorithmically generated for transformation of any
RWD source.

SuperLineage supplies the accuracy, completeness and traceability that FDA asserts is essential for trusting RWD in regulatory submissions.
Learn how to use SuperLineage to trust RWD in regulatory submissions






